Unsaturated Polyesters
Properties
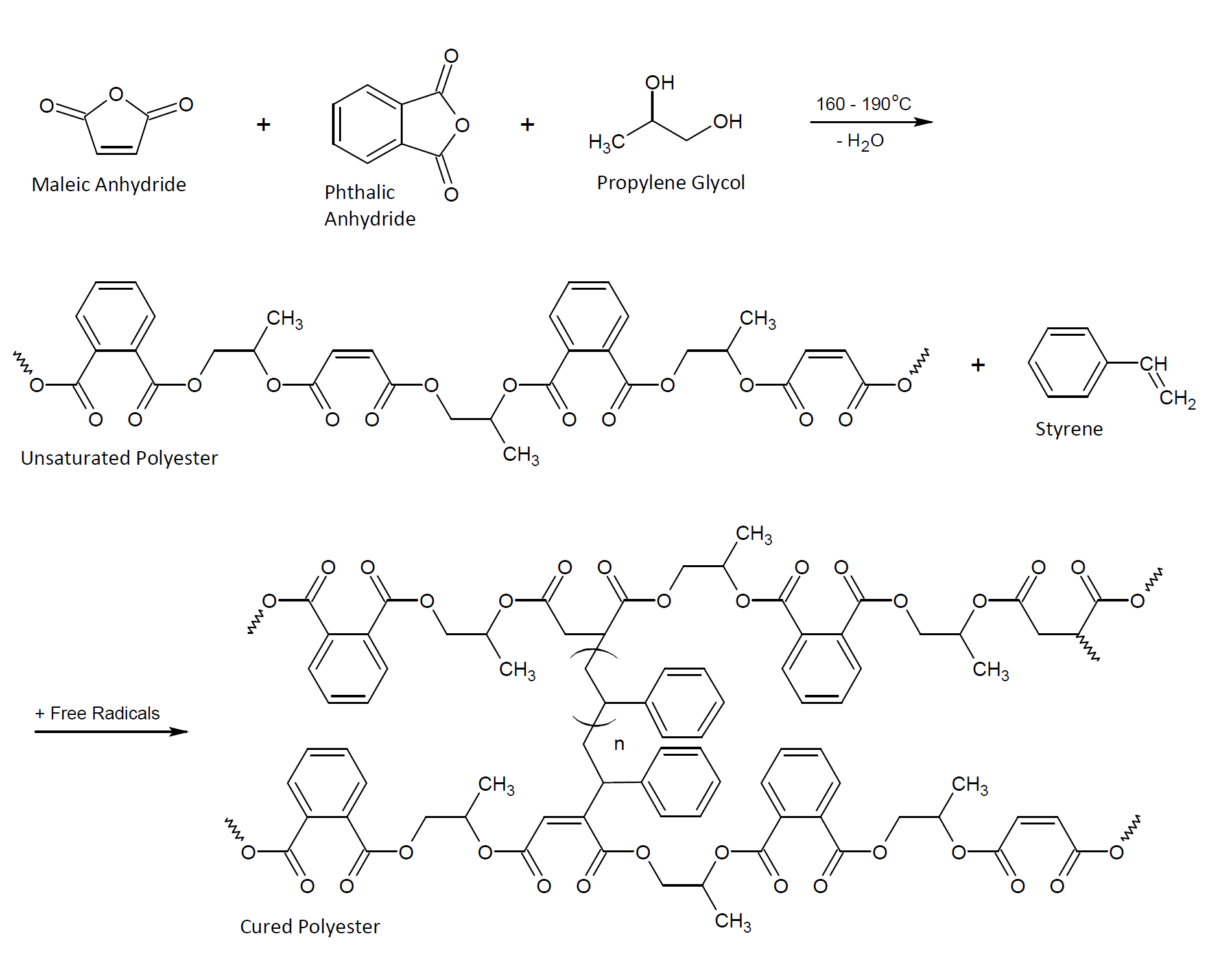
Unsaturated polyesters are the third-largest class of thermoset molding resins. They are produced by the condensation of a diol with a blend of saturated and unsaturated anhydrides. The condensation products (reactive resins) form very durable structures and coatings when cross-linked with reactive vinyl monomers such as styrene.
The properties of the cross-linked resin depend on the types of anhydrides and glycols used and their relative amounts. The majority of commercial unsaturated polyester resins (UPR) are derived from phthalic and maleic anhydride as the saturated and unsaturated component and 1,2-propylene glycol as the diol in the polymer. Many other glycols and acids or anhydrides can be used to taylor the properties of unsaturated polyesters. For example, isophthalic acid (IPA) and terephthalic acid (TA) are sometimes chosen to achieve better thermal and chemical resistance, whereas long-chain aliphatic acids, such as adipic or succinic acid, improve the flexibility but reduce the chemical and heat resistance. Similar improvements in flexibility can be achieved with ether glycols such as diethylene glycol and polypropylene glycol. Monofunctional acids and bases can be employed to tie off excess of hydroxyl and carboxyl groups and to reduce the molecular weight of the final prepolymer. The anhydrides and acids can also be condensed with epoxy resins such as epichlorhydrin or bisphenol A diglycidyl ether. The oxirane rings of the epoxy compounds act as difunctional glycols, that is, they can replace all or some of the polyols. In some cases, carboxylated unsaturated polyesters are caped with glycidyl methacrylates. These resins are sometimes employed in UV curable coating formulations.
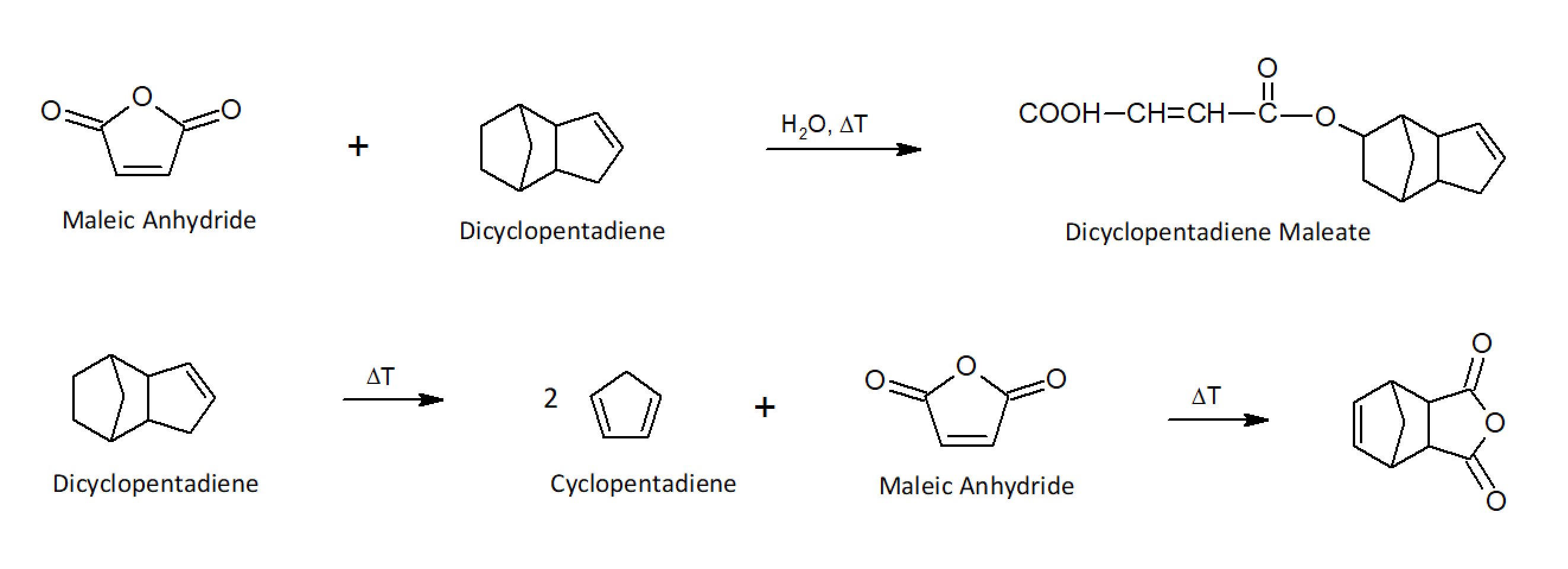
A relative new class of vinyl esters are dicyclopentadiene (DCPD) modified resins. These resins were introduced commercially in the 1980s and have gained significant market share due to their low shrinkage and low styrene content1 DCPD can be reacted with any dicarboxylic acid or anhydride. For example, in the presence of water, dicyclopentadiene and maleic anhydride form dicyclopentadiene maleate. DCPD can also be converted to cyclopentadiene, its monomeric form, which is reacted with the double bond of maleic anhydride via Diels-Alder addition. Both intermediates can be further esterified (polymerized) with a diol such as propylene glycol. These resins provide enhanced thermooxidative resistance, improved processability and low linear shrinkage when crosslinked.
Unsaturated polyesters are typically low-molecular-weight prepolymers. To fully cure them, they are dissolved in a vinyl monomer (and occasionally a solvent2) together with an appropriate organic peroxide3 and poured, sprayed, or molded into the final product and then converted into a thermoset by heating. Many resins can also be room temperature cured when a reducing agent such as a cobalt salt (naphthenate or octoate) or an aromatic tertiary amine is added to the sytem. This compound is often called accelerator or activator. Resin products with an activator are typically two-pack systems whereas heat curable UPRs are typically one-part systems. The cure time and pot-life will depend on the initiator system, its concentration as well as on the type of resin and vinyl monomer and cure temperature.
The most common vinyl monomer is styrene. It functions as a reactive diluent and as a crosslinking agent. When polymerized in the presence of unsaturated polyester, it reacts with the double bonds of the prepolymer but also undergoes homopolymerization. The degree of homo- and co-polymerization depends on the reaction conditions, monomer content, and miscibility of the two resins. In some cases, vinyl cure can lead to the formation of a (semi-)interpenetrating network.4
Due to growing health concern5, styrene has been replaced in many resin systems by other less toxic vinyl monomers such as vinyl toluene, vinyl acetate, methyl methacrylate and allyl ethers as well as by difunctional vinyl monmers such as diacrylates and dimethacrylates. The type of reactive diluent will effect both the cure and the final properties of the polyester.
| Polyols (Glycols) | Saturated Acids / Anhydrides |
Ansaturated Acids / Anhydrides |
Vinyl Monomers |
| Propylene glycol | Phthalic anhydride | Maleic anhydride | Styrene |
| Ethylene glycol | Isophthalic acid | Fumaric anhydride | α-Methylstyrene |
| Diethylene glycol | Terephthalic acid | Methacrylic acid | Methyl methacrylate |
| Epichlorhydrin | Adipic acid | Acrylic acid | Vinyl toluene |
| Bis A diglycidyl ether | Succinic acid | Itaconic acid | Vinytl acetate |
| 1,4-Butanediol | Azelaic acid | DCPD Maleate | Ethylene glycol diacrylate |
Commercial Unsaturated Polyesters
Unsaturated polyester resins are produced on a large scale by a large number of companies including Ashland, Reichhold, and Royal DSM. They are typically sold in liquid form in an unsaturated and reactive solvent, normally styrene. Grades with different viscosities, unsaturation and cure speed are available. DCPD modified resins are produced by Deltech, Polynt, and Dow and others.
APPLICATIONS
Unsaturated polyester resins are mainly used in the production of fiber reinforced plastics and filled plastic products, including sanitary-ware, tanks, pipes, gratings, and high performance components for the marine and transportation industry such as closure and body panels, fenders, boat hulls/ decks and other large glass fiber reinforced plastic articles. Unsaturated polyester resins also find uses in coatings and adhesives.
References and Notes
-
Unsaturated polyester resins have been described in US patent 3,347,806 (Chemische Werke Albert) and 4,029,848 (Dow Chremical).
-
In some cases, conventional solvents such as xylenes are also used. However, most are prepared solely with reactive vinly type monmomers.
-
Common initiators include methyl ethyl ketone peroxide (MEKP), benzoyl peroxide (BPO), and acetylacetone peroxide (AAP).
-
An Interpenetrating polymer network (IPN) consists of two or more polymers which are at least partially interlaced on a microscopic scale but are not covalently bonded to each other. At least one of the polymers is crosslinked in the immediate presence of the other(s). Due to the crosslinking, IPNs can swell, but do not dissolve in solvents, and creep and flow is strongly suppressed.
-
Acute (short-term) exposure to styrene can cause mucous membrane, skin, and eye irritation, whereas chronic (long-term) exposure can effect the central nervous system (CNS) resulting in headache, fatigue, weakness, etc. Styrene is also suspected to cause cancer.5
-
The Department of Occupational Medicine at Aarhus University in Denmark concluded that styrene may cause cancer in humans.